-------------------------------------------------------------------
1. Introduction
There is rapid progress in microfluidic research in past decades. It utilizes the unique physical phenomena that can be realized at microscale. For example, laminar flow, mixing by diffusion, efficient heat and mass transfer, high surface to volume ratio. Physical processes can be more easily controlled and harnessed in microscale instruments1. These advantages have been used for chemical reactions at microscale level. Microreactors exhibit numerous practical advantages when compared with traditional batch-scale synthesis which includes the transportation and storage of toxic, explosive or otherwise harmful materials. Microreactors have been used to carry out in-situ chemical tests. The whole aspect of heat management, enabling mass and heat transfer to be extremely rapid, leads inevitably to a higher level of reaction control and reactant manipulation at any one point within a device. In addition, the problems associated with traditional scale-up could be overcome by reactor scale-out producing the required quantity of raw material. Adopting a scale-out philosophy coupled with large-scale microreactor fabrication technology, it is possible to see how the optimization of reaction conditions on a single device could be extended, allowing multiple numbers of single units referred to as parallel scale-out. Thus one can conclude that microreactors applied to the field of chemical and biochemical synthesis offer greater reaction control and selectivity, which in turn can be optimized through a scale-out methodology creating a safe and efficient approach to chemical discovery and production.2
This project report describes some of the important properties of microfluidic device which can be used for chemical reactions (e.g. nanomaterial synthesis and DNA amplification reactions).
2. Mixing, heat transfer and synthesis in microreactors
2.1 Mixing: For a chemical reaction to happen contact between reacting chemical species must be realized and it happens by good mixing. In microfluidic devices the flow is laminar and the mixing mainly occurs by diffusion in contrast to fast convective process in turbulent flow system in macrosystems. Effect of mixing on the extent of a reactant and product distribution is crucial for a chemical reactor. Mixing in microfluidic devices can be classified into passive and active mixing. Passive mixers don’t rely on external energy; the mixing process depends on diffusion or chaotic advection. Active mixtures use external energy which causes disturbances and enhances mixing. External disturbance effect can be achieved by using pressure, temperature, electrohydrodynamics, dielectrophoresis, electrokinetics, magnetohydrodynamics and acoustics.3 Passive mixtures rely on geometric properties of the channel or fluidic streams to maximize the area over which diffusion can occur. On the other hand active mixers rely on time-dependent perturbations of the fluid flow.1 Active mixtures structures are complicated, fabrication of such mixtures is difficult, require external power. On the other hand simple passive micromixer structures are robust, stable in operation and easily integrated in a more complex system.3 Therefore passive mixers have found the widest use in synthetic applications1. Some example designs of mixers are described in Fig. 1.
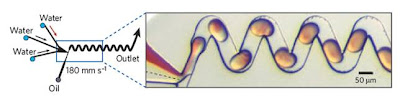
In recent studies droplets are being used as a very promising reaction vessel themselves. Droplets are produced by injecting laminar streams of aqueous reagents into an immiscible carrier fluid (e.g. oil). These droplets are separated from each other and from the wall of the channel, each one acts as an individual reaction vessel. (Fig. 2) Separation from channel wall prevents a significant variation in the yield, efficiency and product distribution of a reaction along the channel because of the hydrodynamic velocity profile. Along with this advantage of using droplets, the mixing can be efficient, rapid and reagent can be transported without dispersion by using generating chaotic mixing within droplets by folding, stretching, and reorienting fluid using suitable channel geometry. Consequently, mixing is rapid and reagent transport occurs with no dispersion. When such features are combined with the ability to combine, split and sort droplets, microfluidic systems are used in high-throughput synthesis due to high sample throughput and kinetic measurements due to low sample requirements and negligible dispersion.1
Figure 2: Droplet formation in microfluidic device and chaotic mixing in droplets: Separated droplets are produced when two different phase liquid is introduced into microchannels in controlled way. Such droplets are passed through zig-zag channel which helps for efficient mixing.
2.2 Heat Transfer: Another important property of microfluidic devices is high thermal transfer efficiencies. Microfluidic devices work on small volume of samples which has small thermal mass and because of this and high surface-to-volume ratios heat transfer in such device is very fast. This phenomenon has been utilized to perform exothermic and/or high temperature reactions in an efficient and controllable (isothermal) manner in. The laminar flow profile in the reduced dimensions of microdevices enables accurate heat and mass transfer characterization.4
2.3 Synthesis in microfluidic system: Several synthesis reactions have been performed in microfluidic devices. Microfluidic environments have been shown to provide for efficient temperature control especially in exothermic reactions and thus reaction control. Both homogeneous and heterogeneous reactions are possible in microfluidic platform. In addition to homogeneous reactions, the large surface-to-volume ratios characteristic of microfluidic reactors provide unique environments for performing heterogeneous chemistry. Gas-liquid-solid hydrogenation reactions in microchannels have been successfully performed with enhanced efficiency using microfluidic. Synthesis reactions in microfluidic system are possible because of good mixing, temperature control etc.1
The ability of microfluidic device to rapidly mix reagents, provide homogeneous reaction environments, continuously vary reaction conditions, and add reagents at precise time intervals during reaction progression has made it an attractive technology for a myriad of applications. Over the past decade, microfluidic devices have enabled screening of a variety of reaction conditions by systematically varying flow rates, temperature, and reactant concentrations in order to optimize the quality of the resulting products using very small amounts of reagents.4 Much of the interest in using microfluidic systems for synthetic applications lies in their ability to perform rapid and controllable mixing. This, combined with manipulation of variables such as temperature, concentration gradients and pressure, dictates that continuous-flow processing on the microscale can be used to synthesize species of specific yet variable characteristics.1
3. Nanoparticle synthesis and PCR in microfluidic platform
3.1 Nanoparticle Synthesis: Nanoparticles (NPs) have been widely used in diverse areas such as electronics, energy, textiles, biotechnology and medicine, bio-imaging, bio-sensing, and gene and drug delivery. Shape and size determine the optical and electronic properties of nanoparticle.5
Nanoparticle synthesis consists of two events. First one is nucleation of solute molecules and second is growth of seed in which the seeds capture dissolved solutes. The step must be shorter than the second one for nanoparticle synthesis. And nucleation and growth should occur in an environment in which chemical state functions are precisely controlled. If these conditions are not met, the size of critical nuclei and growth rates will vary according to location, and result in a distribution of particle sizes.1 Recent studies have demonstrated that microfluidic reactors drastically outperform macroscale systems in the direct production of nanoparticles. Using simple flow regimes whereby component streams are mixed at low Reynolds numbers and in continuous flow, variations in reaction residence times, temperatures and reagent concentrations are used to control average particle size, while sample size distributions are minimized through a reduction in residence-time distributions and precise control of chemical state functions.1
Microfluidic technologies offer a variety of advantages over conventional technologies for the synthesis of nanoparticles. Nanoparticle synthesis in microfluidic devices also get advantages from small volume and then by reducing the cost, enhanced heat and mass transfer because of the large surface area to volume ratio, ability to monitor and control fast reactions and efficient mixing which are available due to the miniaturization of chemical reactors.1
It is possible to produce specific nanoparticles in microfluidics platform by controlling temperature, concentration gradients, pressure and rapid and controllable mixing. Because both mass and thermal transfer is rapid, temperatures may be defined with precision or varied on short timescales. Additionally, reagents can be rapidly and efficiently mixed to ensure homogeneous reaction environments, while allowing for additional reagents to be added at predefined times.1
Different types of nanomaterials (e.g. cadmium sulphide, cadmium selenide, palladium, silver, gold, copper, titania and CdSe–ZnS core–shell nanoparticles) have been synthesized in microscale. An example of nanoparticle synthesis in microreactors has been described below.
Figure 3: The microfluidic reactor for the nanoparticle synthesis. a) The optical image of this microfluidic reactor shows three parts, the Y mixer with inlet 1, inlet 2 and the Y mixer, the reaction channel with width of 60 µm and length of 30 cm, and the quenching channel with width of 120 µm and length of 15 cm. The depth of all channels is 600 µm. b) TEM images of the worm/chain-like Pd nanoparticles synthesized the microfluidic reactor.5
The device presented in figure 3 was used to synthesize worm like and chain-like shaped metallic nanoparticles at room temperature and under inert atmosphere. PdCl2 tetrahydrofuran (THF) solution was pumped into the reactor from inlet 1 at lithium hydrotriethylborate THF solution containing sulfobetaine (SB12) was pumped inlet 2. Reduction of PdCl2 occurred instantly upon mixing after the two reagent streams met at the Y mixer. Upon completion of the reaction and the growth of NPs in the reaction channel, the black colloidal solution was further treated by long-term stirring, or sonication, or quenched immediately using a quenching solution (2 mL acetone in 67 mL THF), that was pumped through the quenching inlet. Then the quenched product was collected at the outlet of the MFR in a glass bottle, and the Pd NPs allowed settle down. The precipitate was then washed twice in pure THF to remove most of the waste salts and some of surfactants. After drying, a black fine powder of Pd was obtained.5
3.2 Polymerize Chain Reaction (PCR): The PCR is an enzyme-mediated process where a single DNA molecule can be rapidly amplified into many billions of molecules. This technique has become a standard technique to selectively and exponentially amplify trace amounts of DNA.4
Typically, PCR consists of a series of 20-40 repeated temperature cycles consisting of three steps. At first DNA is denaturated by heating the reaction to 94–98 °C for 20–30 seconds. It causes melting of the DNA template by disrupting the hydrogen bonds between complementary bases and yields single-stranded DNA molecules. In the second step (annealing) the reaction temperature is lowered to 50–65 °C for 20–40 seconds allowing annealing of the primers to the single-stranded DNA template. The polymerase binds to the primer-template hybrid and begins DNA synthesis. In the third step (Extension/elongation step) a temperature of 72 °C is used. At this step the DNA polymerase synthesizes a new DNA strand complementary to the DNA template strand. Thus the DNA polymerase will polymerize a thousand bases per minute.6
PCR in conventional methods have thermal cycles slow and inefficient due to large thermal masses associated with instrumentation. In recent years, PCR in microfluidic devices has attracted a lot of attention because of the potential to dramatically improve the speed, portability, cost, and performance of conventional PCR assays. Thermal mass is the key to fast thermocycling. In microfluidic PCR, the volume of PCR mixture thermocycled is reduced by 1-2 orders of magnitude, and the speed with which thermocycling can occur is be increased.4
A variety of microfluidic systems have been successfully developed to implement microchip PCR. In a continuous-flow PCR formats repeated heating and cooling of the reaction chamber by moving the sample through alternating temperature zones is required which greatly reduces the cycled thermal mass while providing simplified temperature control. In recent years studies have shown that PCR can be confined to a discrete microdroplet or plug surrounded by an immiscible carrier fluid. This mitigates the surface-induced reaction inhibition and cross-contamination arising from large surface to volume ratio of microchannels. The use of discrete droplets also enables much higher throughputs and improves sensitivity, in some cases enabling the detection of single-copy templates.1
Hua et al. have recently described a multiplexed PCR method in microdroplets. The system requires no pumps or valves for fluid manipulation and features a disposable reaction cartridge. Using their device, authors were able to detect the equivalent of a single genome for a methicillin-resistant S. aureus with a remarkable amplification efficiency of 94.7% and reproducibility and sensitivity comparable to conventional bench top real-time PCR instruments but provides many advantages in terms of automation, cost, and time to result. They also performed parallel two-plex PCR amplification of multiple DNA samples for high-throughput multiplexed PCR applications.7
4. Conclusion
Chemical reactions in microfluidic platform take advantages from typical behavior of fluid flow in microchannels. Fluid can be easily controlled at this scale and success of microreactor reactions lies in this fact. Microfluidic systems have been studied for chemical reactions and recent developments in droplet microfluidic have enabled high-throughput screening and kinetic studies of complex chemical and biological systems. Some of the examples of chemical reactions done in microfluidic devices have been described in this report. There are several ways that microfluidic platform could do a lot of progress in future such as genomics, proteomics, drug-discovery and high-throughput screening etc. With all such advantages a question still remains unanswered which is whether microfluidic platform will be able to produce enough chemicals in bulk amount to meet the market demand. Such micro devices use micro to nano-liter volume. Simple calculations have shown that microfluidic platform could be used to produce bulk chemicals by scaling out such devices. Research in microfluidic could go in this direction in next decade.
The advantages come essentially from the thermal, spatial and temporal control possible in such devices, coupled with the capability to monitor reactions in situ while operating if necessary under controlled temperature, pressure and atmospheric conditions. In simple terms, microreactors reduce many of the practical difficulties associated with performing chemical reactions based on traditional methods.
5. References
1. deMello, A J., Control and detection of chemical reactions in microfluidic systems, Nature, 2006, 442, 395-402.
2. Haswell, S.; Skelton, V., Chemical and biochemical microreactors, Trends Anal. Chem. 2000, 19, 389−395.
3. Nguyen, N. T.; Wu, Z., Micromixers-a review. J. Micromech. Microeng, 2005, 15, R1-R16.
4. Roper, M.; Easley, C.; Landers, J., Advances in Polymerase Chain Reaction on Microfluidic Chips, Anal. Chem., 2005, 77, 3887-3894.
5. Song, Y.; Sun, Q.; Zhang T.; Jin, P.; Han, L., Synthesis of worm and chain-like nanoparticles by a microfluidic reactor process, J Nanopart Res, 2010, 12, 2689–2697.
7. Hua, Z.; Rouse, J.; Eckhardt, A.; Srinivasan, V.; Pamula, V.; Schell, W.; Benton, J.; Mitchell, T.; Pollack, M., Multiplexed Real-Time Polymerase Chain Reaction on a Digital Microfluidic Platform, Anal. Chem. 2010, 82, 2310–2316.




No comments:
Post a Comment